Department of Chemistry
CARNEGIE MELLON UNIVERSITY
Introduction to Modern Chemistry (09-105)
EXAM II Name
October 14, 1998 Circle Section A B C D E F G H J K L
Closed book. Show all work.
No equations stored in calculators. No notes.
Don't throw away points using significant figures and units incorrectly!
| H | 2He | ||||||||||||||||
| Li | Be | B | C | N | O | F | 10Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | 18Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | 36Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | 54Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | 86Rn |
| Fr | Ra | Ac* | Rf | Ha | Sg | Bh | Hs | Mt | 110 | 111 | 112 |
| Qe = 1.6022 € 10-19
C (unit of charge) 1 D (debye) = 3.336 € 10-30 C . m 1 pm =10-12 m |
|
| 1. /25 | |
| 2. /25 | |
| 3. /25 | |
| 4. /25 | |
| /100 | |
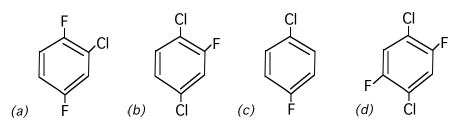
1. Arrange the following in order of increasing dipole moment, lowest first. Indicate the sequence by the letters assigned to each structure. (Correct sequence equals full score. One structure out of sequence equals half score. More than one out of sequence gets no score.)

(d) < (c) < (a) < (b)
Orthoperiodic acid, with iodine as the central atom, has the formula OI(OH)5. Will the pK of its hypothetical analog with astatine, OAt(OH)5, be greater, lesser or the same as that for OI(OH)5?
greater
What is the oxidation number of iodine
in orthoperiodic acid?
7
Will periodic acid (HO)IO3 (in which iodine is again central) be a stronger or weaker acid than orthoperiodic acid?
stronger
The most stable compound between oxygen and fluorine has the formula OF2. What is the oxidation number of oxygen in this compound?
+2
2. The hydrogen-potassium bondlength in HK is 152 pm. The dipole moment is measured to be 6.13 D (debyes). Calculate the fractional ionic character of the HK bond.
m = Q.r
m(ionic) = (1.60 X 10-19 C)(152 X 10-12 m)
= 2.43 X 10-29 C.m
--> (2.43 X 10-29 C.m)/(3.34 X 10-30 C.m/D) = 7.29 D
m(observed)/m(ionic) = 6.13 D/7.29 D = 0.841 (fractional ionic character)
What is the oxidation number of potassium in this compound?
+1
If the fractional ionic character of barium sulfide, BaS, were found to be exactly 0.50, which of the following would best be cited as the complete, preferred Lewis structure?
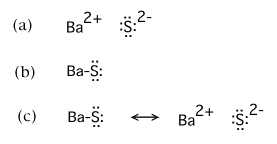 |
(c) is the correct choice |
This past Monday, the Nobel Prize in Medicine was announced and concerns the discovery of the many roles played by nitric oxide (NO) in physiological systems. What is the complete, preferred Lewis structure for NO?
| structure is |
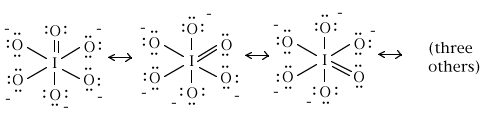
3. As mentioned in problem 1, orthoperiodic acid, with iodine as the central atom, has the formula OI(OH)5. When it is completely ionized, the IO65- ion is produced. Draw the complete, preferred Lewis structure for this latter ion, the orthoperiodate ion.
 |
||
What is the iodine-oxygen bond order for each of the six iodine-oxygen bonds?
14 electrons / 6 iodine-oxygen links = (7/3) electron per bond on average
bond order = (1/2)(7/3) = 7/6 = 1.17 for each of the six bonds
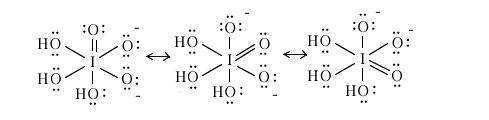
When the acid is incompletely ionized, one of the intermediate species is the ion [O3I(OH)3]2- still with iodine as the central atom. What are the iodine-oxygen bond order for each of the six iodine-oxygen bonds in this ion?
 |
||
The three iodine-oxygen bonds involving the OH are each of bond order 1.00 and
the three (resonance) iodine-oxygen bonds involving O- are (8 e-/3 bonds)(1/2) = 4/3 = 1.33 each
4. Below is the structure of nicotine. For the three angles indicated by the italic numbers 1, 2, and 3, what are the best values you should be able to give?
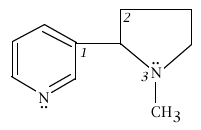 |
angle 1 is ____< 120o____________ angle 2 is ______109.5o_________ angle 3 is ______< 109.5o________ |
What are the molecular geometry names and the best estimates that you should be able to give for the FXF bond angles in each of the molecules below (where X just represents the central atom)?
molecule |
molecular geometry |
FXF angle |
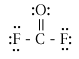 |
|
|
CF4 |
tetrahedral |
109.5o |
NF3 |
trigonal pyramid |
< 109.5o |
XeF2 |
linear |
180o |