| These "slides" represent highlights from lecture and are neither complete nor meant to replace lecture. It is advised not to use these as a reliable means to replace missed lecture material. Do so at risk to healthy academic performance in 09-105. |
Induced dipoles
Polarizability
Van der Waals interactions
Permanent dipoles
Temporary (induced) dipoles
Boiling points, melting points, densities
Effects of size
Effects of shape
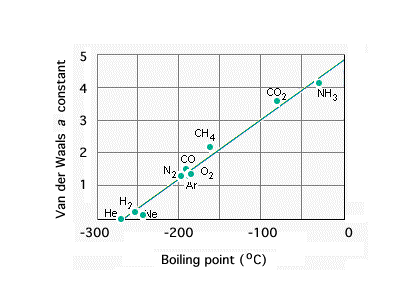
Obviously, you know water's boiling point is way off following the prediction shown.
The explanation is a extraordinary phenomenon called hydrogen bonding.