| Lecture
#29 |
Sections from Chapters 12 and 13 (See
Syllabus =  in Main Index) in Main Index) |
| CURMUDGEON
GENERAL'S WARNING. These "slides"
represent highlights from lecture and are neither
complete nor meant to replace lecture. It is
advised not to use
these as a reliable means to replace missed
lecture material. Do so at risk to healthy
academic performance in 09-105. |
|
| |
Intermolecular Interactions
- Liquids and Solids
Hydrogen Bonding
- Molecular Basis of Solubility
|
| Boiling point effects owed to hydrogen bonding |
 |
| Multiple hydrogen bonds |
 |
| A comparison of melting points of two similar looking
compounds, both are benzene rings with
"hydroxy" and "nitro" substituents in
different positions. For comparison, below on the left,
the "hydroxy" group has been replaced by a
"methyl" group. |
 |
| Melting points are also affected by hydrogen bonding. |
 |
| The melting points of these two structural isomers of
nearly identical electronic volumes differ substantially.
Why? |
 |
| An illustration of an "intramolecular"
hydrogen bond within a molecule (rather than between
molecules). |
 |
| Some definitions relevant to discussions on
solubility. |
 |
| What effects determine solubility? |
 |
| The dependence of the solubilities of different
alcohols on their molecular structure |
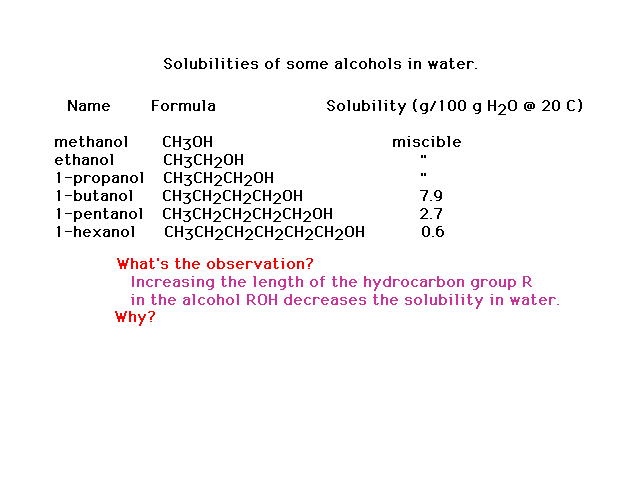 |
| Interpretation of alcohol solubilities in terms of
molecular structure and intermolecular interactions |
 |
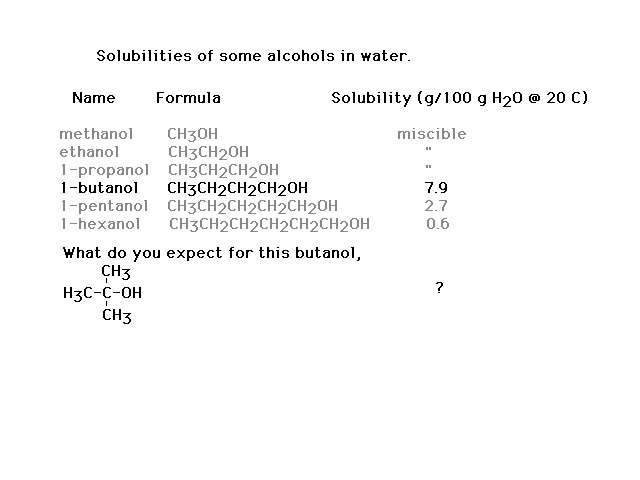
| Another comparison to make. |
 |
| Vitamin A shown here is essentially a nonpolar
molecule. It is very polarizable because of its
"size" (and also because pi electrons are very
polarizable, a detail we haven't worried about and will
not). Consequently, it is not very soluble in water but
quite soluble in a nonpolar solvent such as
"fat" (which is mostly hydrocarbon-like in
nature). |
 |
| In contrast to Vitamin A, you can see here from the
structure of Vitamin C that extensive hydrogen bonding
with solvent water molecules should be possible,
explaining why it is a water soluble vitamin. |
 |
| Acetic acid, as a small, polar molecule capable of
hydrogen bonding with water is very soluble in water. You
might reasonably expect it to be insolube in nonpolar
solvents. However, a subtle phenomenon causes acetic acid
to be soluble in nonpolar solvents as well. See below. |
 |
| Acetic acid can effectively "dimerize",
form a double structure held togeter very effectively by
a geometrical arrangement that accommodates double
hydrogen bonding. The resulting structure has no dipole
moment. The "dimer" can then serve as a solvent
in nonpolar solvents. |
 |



