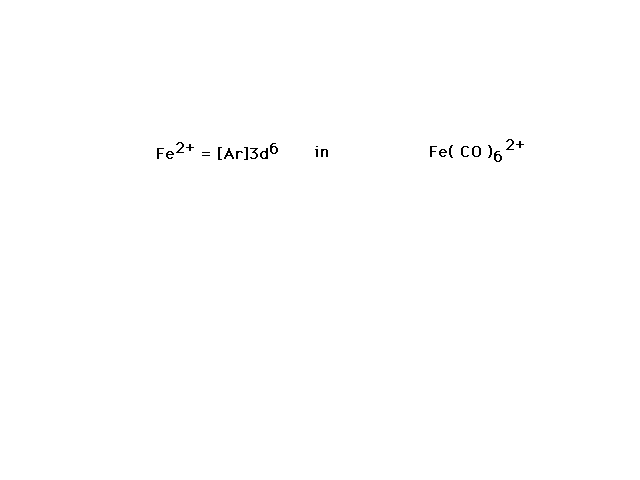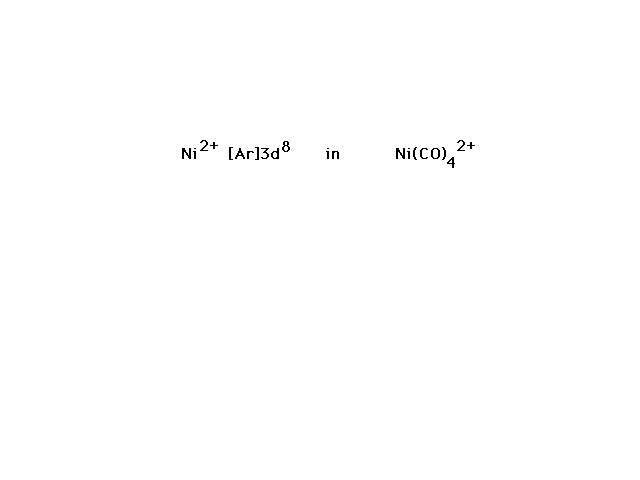| Lecture
#36 December 5, 2005 |
| |
| CURMUDGEON
GENERAL'S WARNING. These "slides"
represent highlights from lecture and are neither
complete nor meant to replace lecture. It is
advised not to use
these as a reliable means to replace missed
lecture material. Do so at risk to healthy
academic performance in 09-105. |
|
| Lecture outline |
Transition Metal Complexes
- Ligand Bonding to central ion's hybrid
orbitals
|
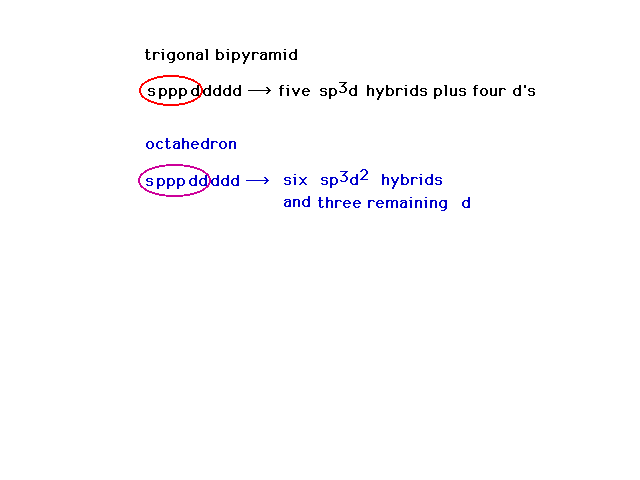
| Crystal field theory told us nothing about the actual
bonds. To begin our discussion of bonding in transition
metal complexes, we return to consideration of hybrid
atomic orbitals. Here is a review of hybrid atomic
orbitals for atoms which can have expanded octets as
would be the case for our transition metal ions. |
 |
| The highest occupied atomic orbital in Cr3+
and the lowest unoccupied orbitals are shown. The d2sp3
hybrids can be constructed from appropriate choices here. |
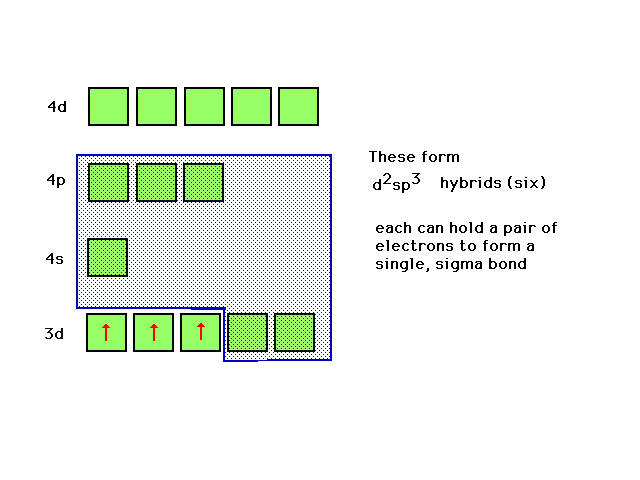 |
| A review of d2sp3 hybrid atomic
orbitals |
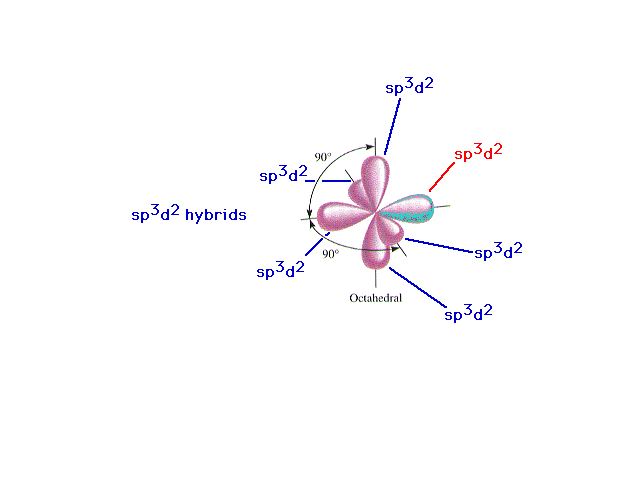 |
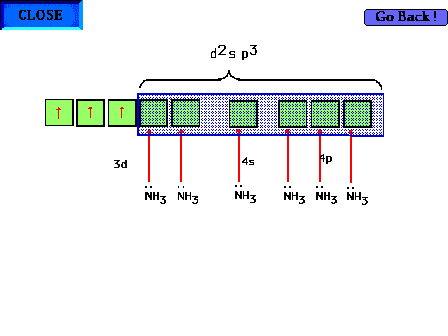
| The ammonia ligands bind to the transition metal ion.
In theory, the bond forms in the region of overlap
between the transition metal ion's hybrid atomic d2sp3
orbital and the ligand's hybrid sp3 atomic
orbital. Two electrons go here, both from the ligand, to
form a sigma bond. |
 |
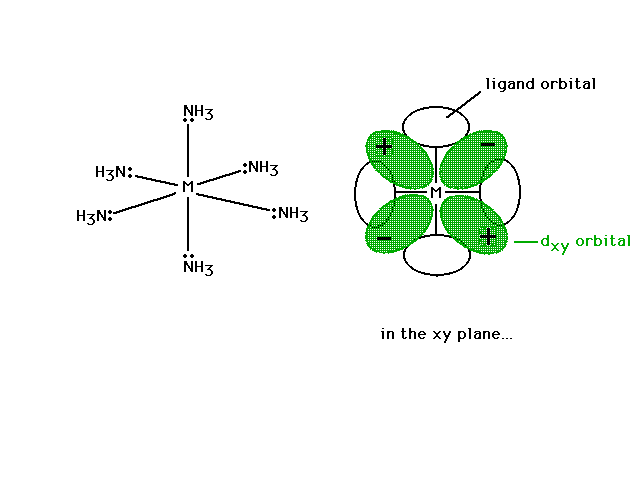
| Looking in just the xy plane where the sigma bonds to
four NH3's are located as is the dxy
non-bonding orbital. The ligand atomic orbital which
would overlap with the transition metal hybrid orbital (a
non-bonding interaction) is shown on the x- and y-axes. |
 |
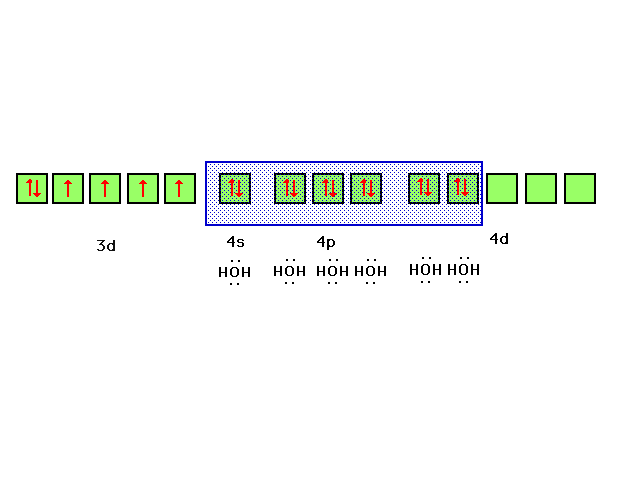
| Fe(H2O)62+ is our
next example. |
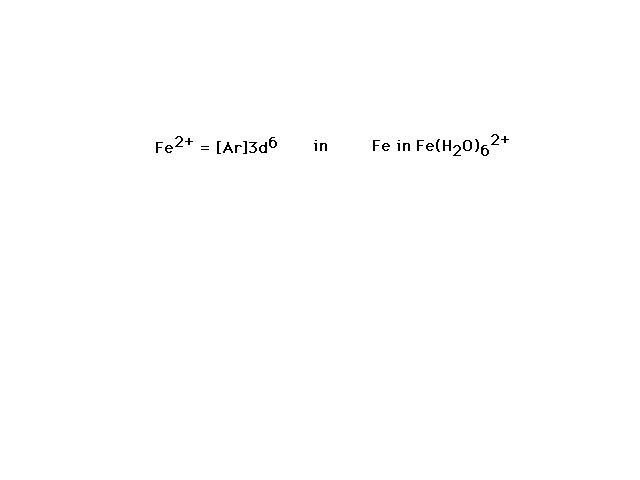 |
| If we allow iron's six valence electrons to occupy
the 3d orbital as indicated, each water will contribute a
lone pair of electrons to a bond between its sp3
orbital and iron's d2sp3 orbital
formed as indicated |
 |
| Fe(CO)62+ is our next
illustration. Since CO is a strong field ligand, we
should not be surprised to see a low-spin complex ion
electron configuration show up by necessity. |
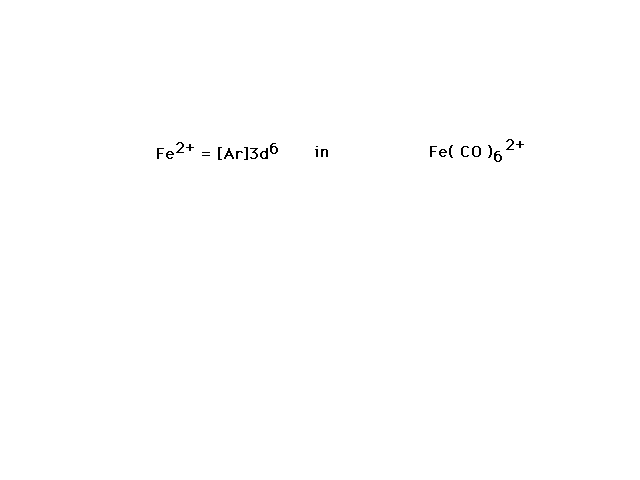 |
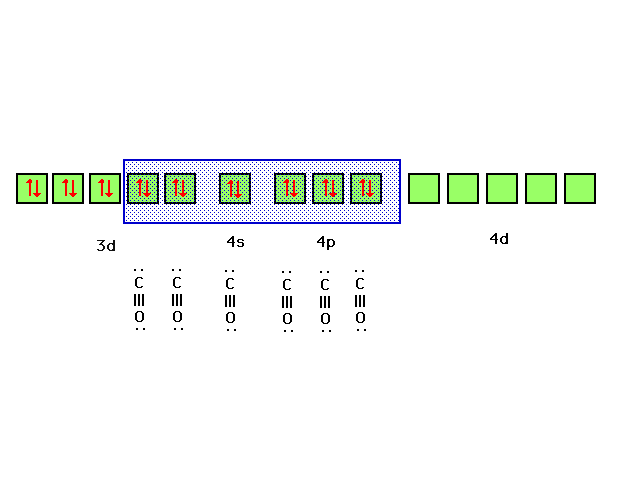
| Bonding of the CO ligands to the d2sp3
hybrid orbitals chosen as illustrated is consistent with
the complex ion being diamagnetic. |
 |
| Looking in just the xy plane, we see that the
electron in the dxy nonbonding orbital is
repelled by chlorine's lone pair in its p-orbital,
weaking Cl's bond to the metal. |
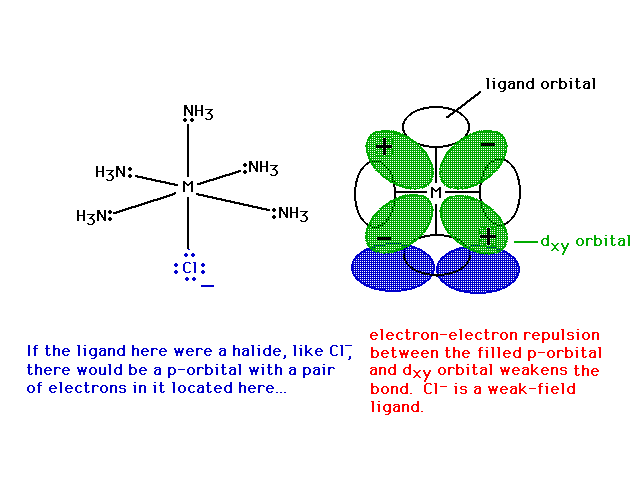 |
| Can we now explain why CO (and CN-) are strong field
ligands? |
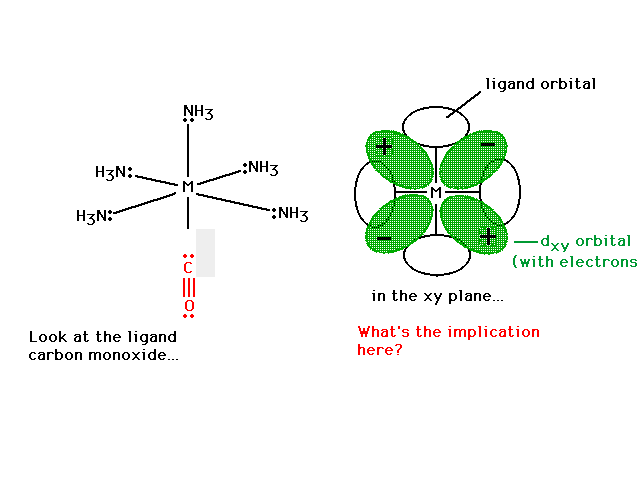 |
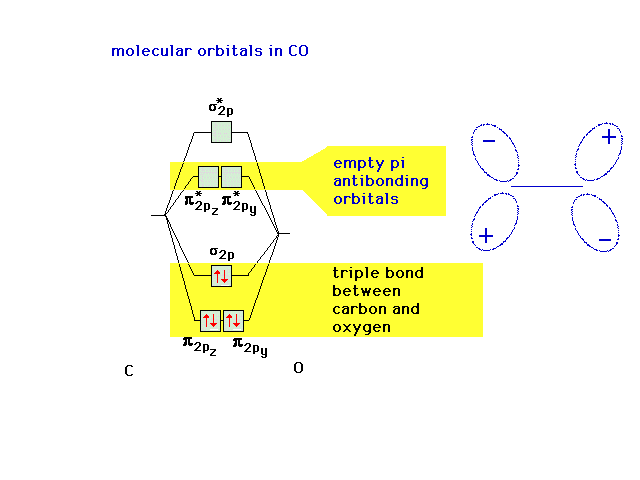
| Let's remind ourselves of the underlying valence
electron structure associated with CO, including the empty
anti-bonding molecular orbitals. |
 |
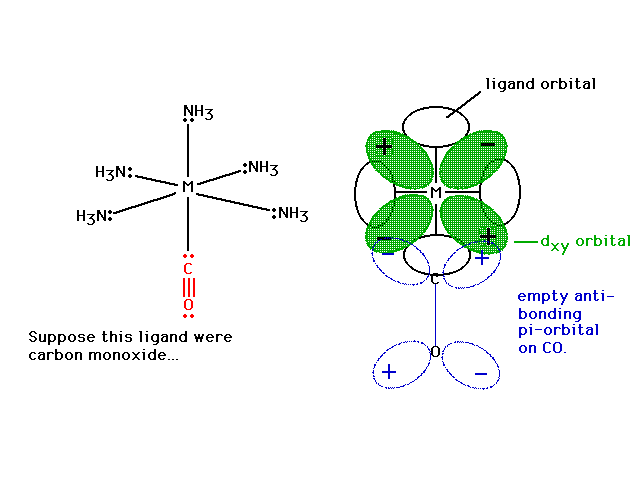
| With CO sigma bonded to the metal just as NH3
and Cl- were, the CO has its empty
pi-anti-bonding orbital placed so that a delocalized
molecular orbital in conjunction with the metal's dxy
becomes possible. Note the constructive interference
possibility. |
 |
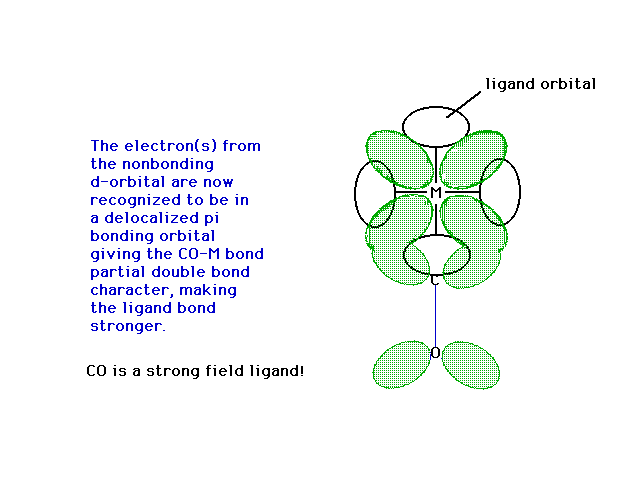
| Here, then is the delocalized orbital into
which the previously though-to-be-non-bonding electron is
placed, giving the CO bond to the metal some partial
pi-bond character...making it a stronger bond! |
 |
| ZnCl42- is the next example. |
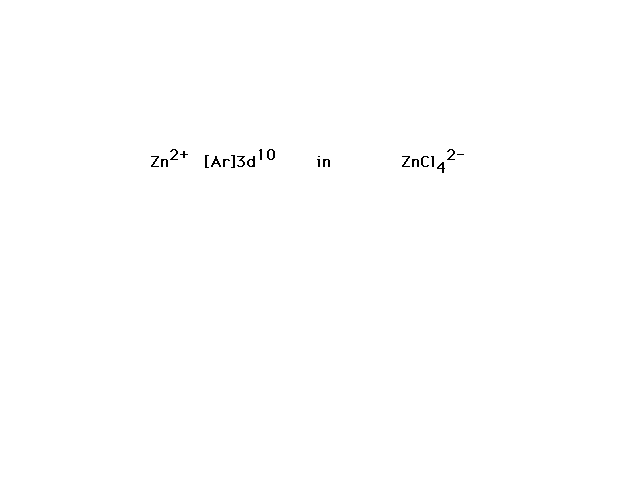 |
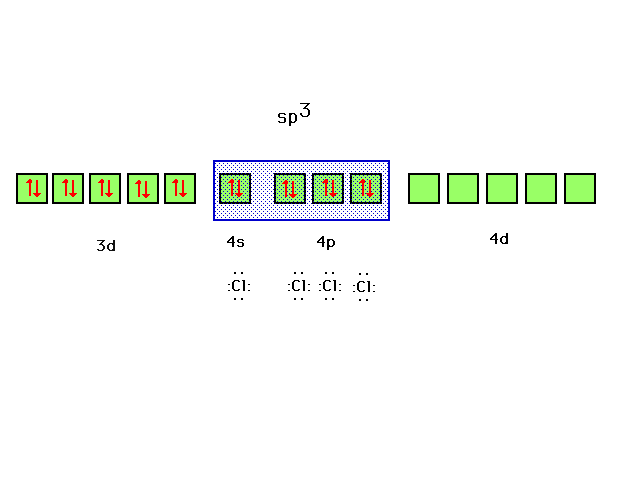
| Ten valence electrons fill the zinc 3d orbital. The
four ligands, each contributing a lone pair to bond
formation, utilize the hybrids formed from Zn's next four
orbitals, the 4s and the three 4p's corresponding to sp3
hybrids and the observed tetrahedral geometry for
complexes of this type. |
 |
| Ni(CO)42+ is our last
illustration. |
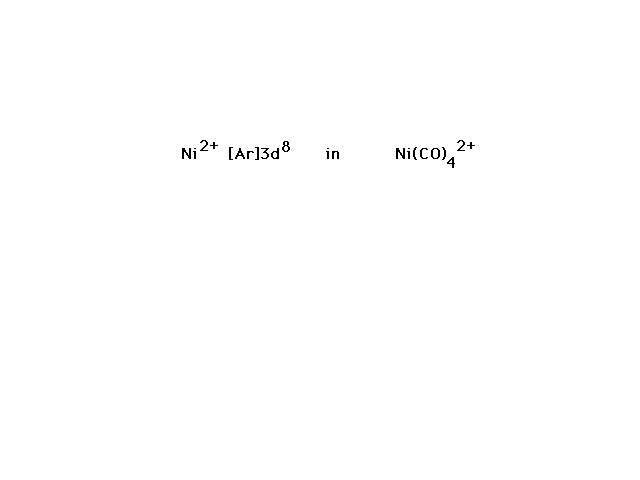 |
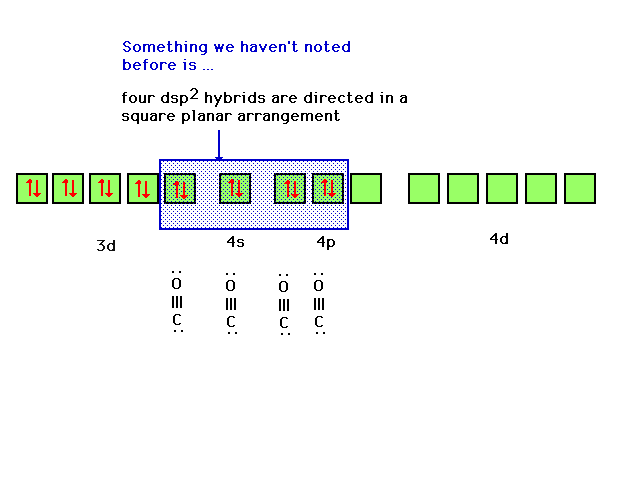
| The arrangement which is consistent with the observed
properties of the complex (geometry and magnetic
properties) and other complexes similar to it arises from
use of four Ni orbitals that hybridize to give dsp2
hybrids. Although we have not discussed this combination
before, it does correspond to four equal orbitals
arranged in a square planar distribution. |
 |