| *Equivalents of NaOH = Volume x concentration. mequiv. = mL x 1.0 M (i.e. mequiv. = 10-3 x equivalents.) |
Graph of the calculated results: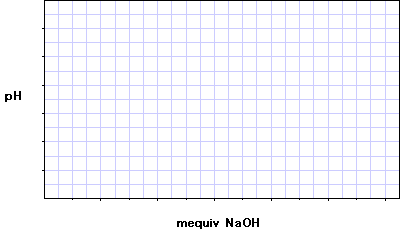 The acid concentration = The pKa = From Table 2.6 of Campbell, the acid is |
|
This page is available as a ![]() PDF image.
PDF image.
![]() Back to the Calculations & Graphing Index
Back to the Calculations & Graphing Index