| Lecture
#11 |
| |
| CURMUDGEON
GENERAL'S WARNING. These "slides"
represent highlights from lecture and are neither
complete nor meant to replace lecture. It is
advised not to use
these as a reliable means to replace missed
lecture material. Do so at risk to healthy
academic performance in 09-105. |
|
| Lecture Outline |
Molecular Structure
Lewis Structures
Formal charge and preferred structures
Exceptions to the Octet Rule
Incomplete octets
Odd electron numbers
Expanded octets (hypervalency)
Examples
Resonance
Equivalent preferred contributors
Bond order
|
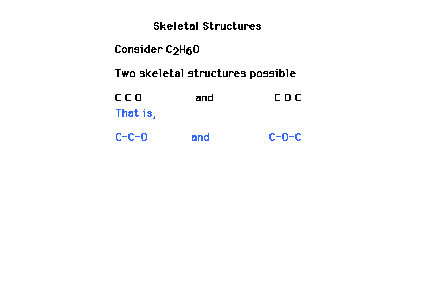
| One of the first steps in constructing a Lewis dot
structure is to decide on the "skeletal"
arrangement of key atoms. Hydrogen and fluorine are
(almost) always on the outside and thus not part
of the skeleton. |
 |
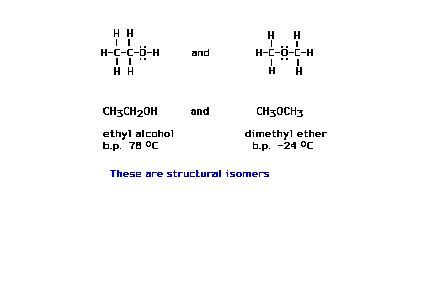
| Here are two different chemical species, each with
exactly the same molecular formula. They are the fairly
common substances ethanol and dimethyl ether. (b.p.
abbreviates boiling point) |
 |
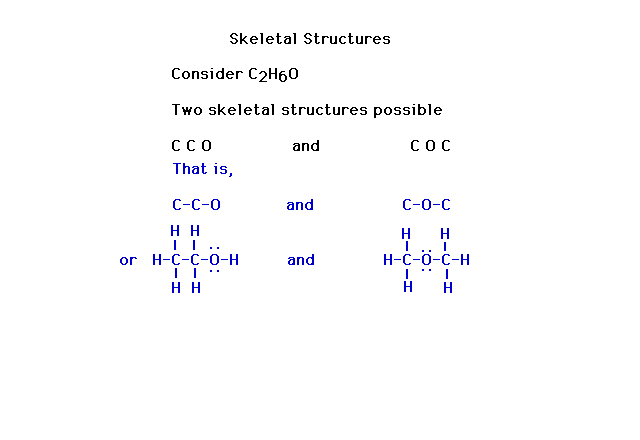
| Without skeletal information, there can be more than
one result in figuring out a Lewis electron dot
structure. |
 |
| Here we introduce the term "structural
isomers" using the above illustration. |
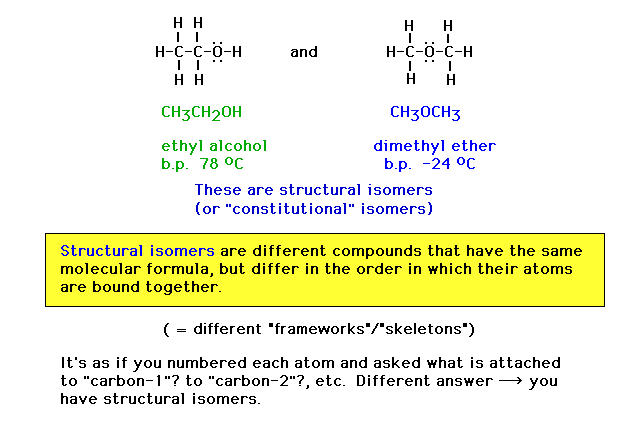 |
| Note that Lewis dot structures are an accounting of
where valence electrons are likely to be found. The
structures are very useful for giving quick information
about molecular structure, but do not represent what a
rigorous theory tells us about valence electron
distributions. |
 |
| First exception to be discussed is what had
previously been mentioned with respect to hydrogen; the
lightest elements often do not complete their octets. |
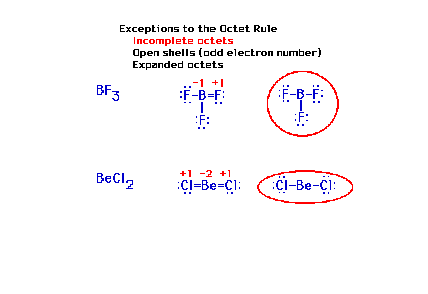 |

