| Lecture
#14 |
| |
| CURMUDGEON
GENERAL'S WARNING. These "slides"
represent highlights from lecture and are neither
complete nor meant to replace lecture. It is
advised not to use
these as a reliable means to replace missed
lecture material. Do so at risk to healthy
academic performance in 09-105. |
|
| Oxoacid strength and molecular structure are
discussed a bit in pp. 691-3, but is mostly from lecture
material. |
Dipole moments
Partial ionic character
Molecular Basis of Acid Strength
Acids
pK values
Oxoacids (continued)
|
| A purely covalent bond, with perfectly shared valence
electrons, has no "polarity" or separation of
charges. The degree to which a bond has polarity can be
expressed by a dipole moment, defined
here (but not in the text.). |
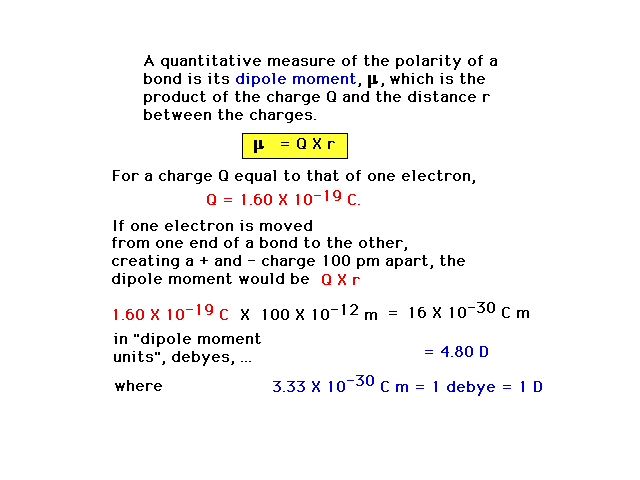 |
| Calculating the "percent ionic character"
or "partial ionic character" for the diatomic
molecule HF using the measured dipole moment and the
measured bond length. |
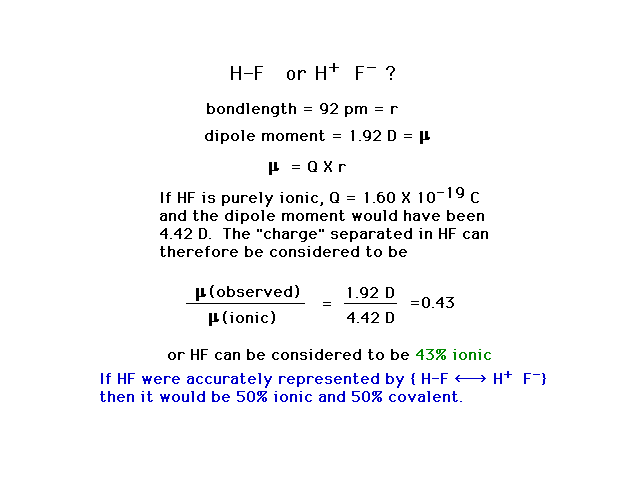 |
| The percent ionic character for the hydrogen halides.
You should be able to calculate the %'s from the various
bondlengths and dipole moments. Note that the percent
ionic character decreases as the halogen becomes less
electronegative, leading to an almost purely covalent
bond (covalent = 0% ionic) for HI.You might want to try
one of these. Percent ionic character is not in our text. |
 |
| As an important application of some of the influence
of electronegativity and of resonance, we will now look
at the effect of molecular structure on the
"strengths" of acids. (This is in Chapter 10
pp. 328-9 to a partial extent only.) What's an acid? |
 |
| A measure of acid strength is its pK value. In
this course, we will not work out any numerical
calculations, but just use the known values to make
qualitative comparisons of acid strength. |
 |
| We will look almost entirely on a category of acids
known as oxoacids (or oxacids) and how the strength of
the acid is understood from the structure of its
molecule. |
 |
| The strengths of some oxoacids are determined by the
electronegativity of nearby atoms. |
 |
| Nearby oxygens affect acid strength in a very
regular way. |
 |
| Another comparison of related oxoacids. (We
will return to the molecular basis of strengths of
oxoacids after diverting our attention to molecular
geometries for a while.) |
 |
| If the nearby oxygens are other OH groups, those
oxygens barely participate in determining acid strength.
The explanation lies in "resonance
stabilization" of the reactant product as we will
see. |
 |
| Here are shown the Lewis structures for the three
phosphorus oxoacids. (You would need to be given
"skeletal" information to obtain these
structures.) Shown at the bottom is the result of H+
being released; the anion H2PO2-
is stabilized by resonance. |
 |
| The important feature is the ability of the =O to
contribute to stabilizing the product anion as in the
previous slide. |
 |
| Carbonic acid is a carboxylic acid, with its
-COOH group. It's not a strong acid because the central
atom -- the "X" in X-O-H -- is not
electronegative. |
 |
| The effect of an electronegative neighbor to the
central atom can be felt as shown here when chlorine is
substituted for one of the hydrogens on the neighboring
CH3 group. |
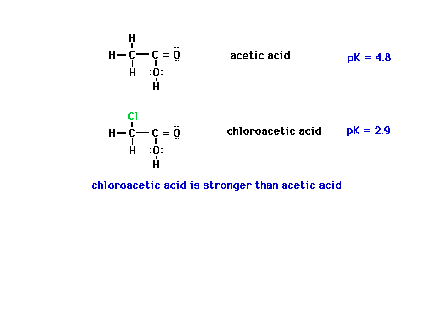 |
| And, in this situation, three fluorines are more
effective than one fluorine. |
 |