| Lecture
#17 |
 VSEPR
geometries and Section 13.3
VSEPR
geometries and Section 13.3 |
| CURMUDGEON
GENERAL'S WARNING. These "slides" represent
highlights from lecture and are neither complete
nor meant to replace lecture. It is advised not
to use these as a reliable means to replace
missed lecture material. Do so at risk to healthy
academic performance in 09-105. |
|
| Lecture Outline |
Molecular Geometries
Dipole Moments in Polyatomic Molecules
Rigidity about double bond
Geometrical isomers
Free and restricted rotations about bonds
|
| The simple notion that polarity between two atoms --
the bond dipole moment -- can be vectorally added when
estimating the polarity of a complex molecule frequently
works. Here, in the two linear molecules, the direction
of the resultant dipole is easily argued. The polarities
of dichloroethylenes demonstrate next that the molecules
must be planar. |
 |
| In the dichloroethylene shown, each carbon has a
trigonal planar geometry about it. This alone does not
complete the geometrical description of this molecule
because there remains the question of the orientation of
one of those planes with respect to the other. Measuring
the molecule's dipole moment might resolve this
last question. The dipole moment is exactly zero. The
molecule is nonpolar. |
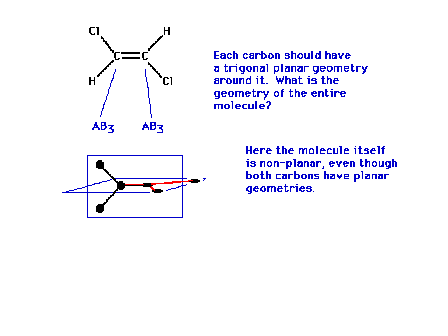 |
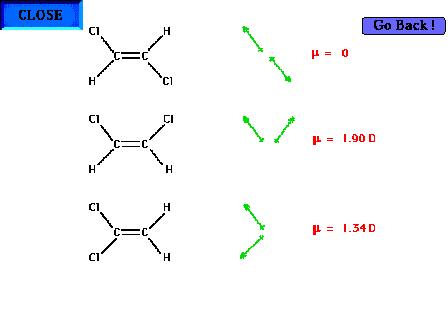
| Three "dichloroethyenes". Given (with an
explanation deferred until the theories of the chemical
bond are discussed) that all three of these double bonded
structures are fairly rigidly planar, we have isomers
whose molecular formulas are all C2H2Cl2.
The bottom molecule is a structural isomer of each of the
other two. Its formula could be written Cl2CCH2
whereas the upper two would be ClHCCClH. . |
 |
| The upper two, because the chlorines are arranged in
distinct geometric locations, are called geometric
isomers (of each other). In the top structure, the
Cl's are across (trans) the double bond from
each other and in the middle structure they are on the
same side of the double bond |
 |
| Dipole moments for each of the three structures based
on our simplification that only the C-Cl bond dipole
moments need be considered. Be aware that the angle
between the pairs of C-Cl bonds is not the same in the
bottom two structures. |
 |
| Here we have the full electrostatic map of the cis-dichloro-ethylene
showing the red-colored negative charge region and the
blue-colored positive region. |
 |
| Chlorobenzene, not surprisingly, is a polar molecule.
The negative end is at the electronegative chlorine, as
expected. The manner in which three dimensional
structures are shown, including a map of electrostatic
(positive = blue, negative = red, neutral = yellow)
potential at the valence surface, is by an illustration
like that here. It was generated by a quantum mechanical
calculation program. |
 |
| Demonstrating the additivity of bond dipole moments
in complicated molecules. We will consider here only the
effect of the carbon-chlorine dipole, ignoring the
carbon-hydrogen dipole. This makes the discussion simpler
and is partially justified by the greater contribution
expected for the C-Cl dipole (large electronegativity
difference) vis-à-vis the C-H dipole. The problem
becomes one of finding the resultant dipole
"vector". |
 |
| Some of the vector additions are almost intuitive.
Two give non-polar (zero dipole) molecules. The third is
polar. |
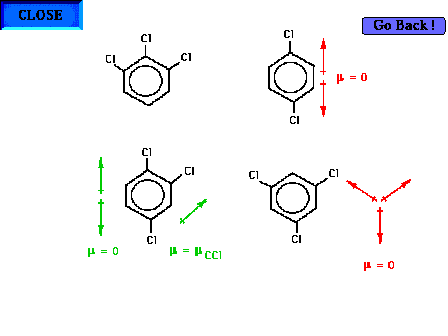 |
| The last is very polar. The vector additions can be
accomplished by doing one pair and then, next, using that
resultant with the final vector. |
 |
| Additionally, a question from Exam II from the Spring
of 2000 will be gone over to give more examples
clarifying the distinction between structural and
geometrical isomerism |
 |



