| Lecture
#21 |
Text: Section
14.4
|
| CURMUDGEON
GENERAL'S WARNING. These "slides"
represent highlights from lecture and are neither
complete nor meant to replace lecture. It is
advised not to use
these as a reliable means to replace missed
lecture material. Do so at risk to healthy
academic performance in 09-105. |
|
| Lecture Outline |
Quantum Theory of the Chemical Bond
Molecular Orbitals
Importance of energy match vs mismatch
Importance of net overlap
Heteronuclear diatomic molecules
|
| In acknowledging the important factors for what leads
to effective molecular orbital (MO) formation, one
feature is the similarity in energy of the atomic
orbitals which are combining (through interference) from
different atoms. Similar energies lead to bonding
molecular orbitals (and antibonding orbitals) that are
highly stabilized (and destabilized), compared to their
atomic orbital (AO) origins. These differences are
mathematically indicated by the weighting coefficients
(c's) in the linear combinations. |
 |
| In heteronuclear diatomic molecules where the atomic
orbitals might differ substantially in energy (such as
for atoms with very different electronegativities or
ionization energies), the bonding molecular orbital is
only stabilized slightly compared to the atomic orbital
of the more electronegative species. Its geometric
shape will also closely resemble that of the atomic
orbital of that same more electronegative atom. This
feature will emerge in the example we discuss soon. |
 |
| Another important feature of atomic orbitals in
determining molecular orbital formation has to do with
relative geometries of the combining orbitals, both in
terms of sizes and in the symmetry with which the + and -
lobes on each atomic orbital combine to give a net
interference effect different than zero. |
 |
| The above factors for influencing the construction of
molecular orbitals are particularly important in looking
a molecules where the valence electrons from two bonding
atoms are in distinctly different atomic orbitals to
begin with. Hydrogen fluoride is an excellent example.
Where will the electrons be in the molecule? How do you
describe the molecular orbitals? |
 |
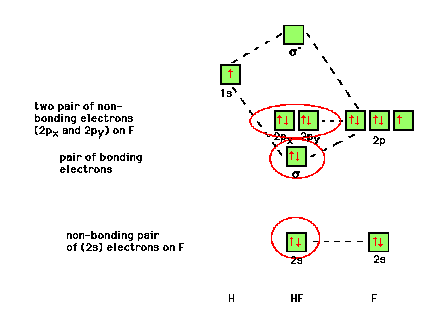
| The 2s atomic orbital on F is so much lower in energy
than the valence 1s atomic orbital on hydrogen that no
molecular orbital will form between them. In HF then, the
first valence electrons go into an orbital that is
indistinguishable from a 2s atomic orbital on F. In
contrast, the 1s(H) and one of the 2p(F) orbitals can
give rise to bonding and antibonding s
orbitals as shown on the left. The contribution from the
more electronegative F dominates the appearance of the
constructed molecular orbital which appears (in yellow)
similar to the fluorine contributing atomic orbital. (The
"exact" result is from a computer calculation.) |
 |
| The flip side of the previous result is that the
antibonding combination is dominated mostly by the more
electropositive species, hydrogen, and its appearance
most closely resembles that of the originating 1s(H) as
shown in yellow. |
 |
| Finally, the 2p(F) atomic orbitals -- which are each
perpendicular to the one previously used to construct the
bonding and antibonding molecular orbitals -- do not give
net interference combinations with the 1s(H) and so
remain unchanged in the HF molecule. That is, they become
non-bonding orbitals in the molecule. |
 |
| Illustrating molecular orbitals (valence electrons
only) in HF whose atoms are very different in
electronegativity. The lowest molecular orbital is
virtually indistinguishable from the 2s atomic orbital on
F (because of the large energy difference with H's atomic
orbital). The s bonding
molecular orbital is close in energy and shape to that of
the F 2px atomic orbital. Fluorine's 2py
and 2pz atomic orbitals have the wrong
symmetry with respect to H's 1s and remain as
"nonbonding" orbitals -- containing lone pairs
on the F. |
 |





