| Lecture
#29 |
| Text: Chapter 16 sections 1,2 |
| CURMUDGEON
GENERAL'S WARNING. These "slides"
represent highlights from lecture and are neither
complete nor meant to replace lecture. It is
advised not to use
these as a reliable means to replace missed
lecture material. Do so at risk to healthy
academic performance in 09-105. |
|
Lecture Outline
|
Intermolecular Interactions
(condensed phases)
- properties of condensed phases
- Molecular basis of solubility
|
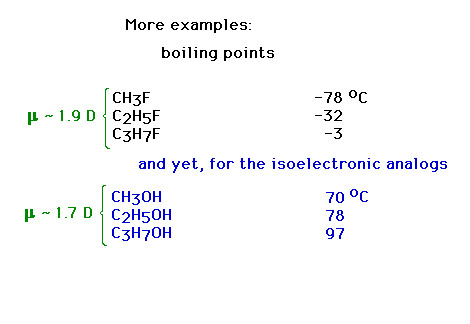
| Examples of London forces and dipole
forces effects |
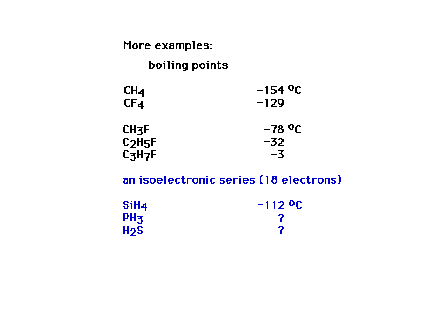 |
| Competition between induced dipole effect and
permanent dipole effect must be considered for this set. |
 |
| Does this comparison suggest a
contradiction? |
 |
Boiling point trends and predictions for
water and for HF.
Obviously, you know water's boiling point is way off
following the prediction shown.
The explanation is a extraordinary phenomenon called hydrogen
bonding. |
 |
| Participants on either side of a "hydrogen
bond" must almost invariably be F, O, or N. |
 |
| The strength of hydrogen bonding relative to the
other interaction strengths we've been looking at. |
 |
| Hydrogen bonding in water (ice). |
 |
| Boiling point effects owed to hydrogen bonding |
 |
| Multiple hydrogen bonds |
 |
| A comparison of melting points of two similar looking
compounds, both are benzene rings with
"hydroxy" and "nitro" substituents in
different positions. For comparison, below on the left,
the "hydroxy" group has been replaced by a
"methyl" group. |
 |
| Melting points are also affected by hydrogen bonding. |
 |
| The melting points of these two structural isomers of
nearly identical electronic volumes differ substantially.
Why? |
 |
| An illustration of an "intramolecular"
hydrogen bond within a molecule (rather than between
molecules). |
 |
| Less interesting, chemically are salt solubilities.
For ionic compounds, as shown here, the attractive forces
are electrostatic and increase with the amount of charge,
like 2+ vs 1+, and decrease as opposite charges get
further apart, as when larger ions are used. The last
salt shown is actually liquid at room temperature and is
an example of an ionic liquid, a newly discovered class
of salts that have tremendous applications anticipated. |
 |
