Lecture
30
|
| (Next moving to Chapter 19) |
| CURMUDGEON
GENERAL'S WARNING. These "slides"
represent highlights from lecture and are neither
complete nor meant to replace lecture. It is
advised not to use
these as a reliable means to replace missed
lecture material. Do so at risk to healthy
academic performance in 09-105. |
|
Lecture Outline
|
Intermolecular Interactions
- Molecular Basis of Solubility
Oxidation Numbers
|
| Some definitions relevant to discussions on
solubility. |
 |
| What effects determine solubility? |
 |
| The dependence of the solubilities of different
alcohols on their molecular structure |
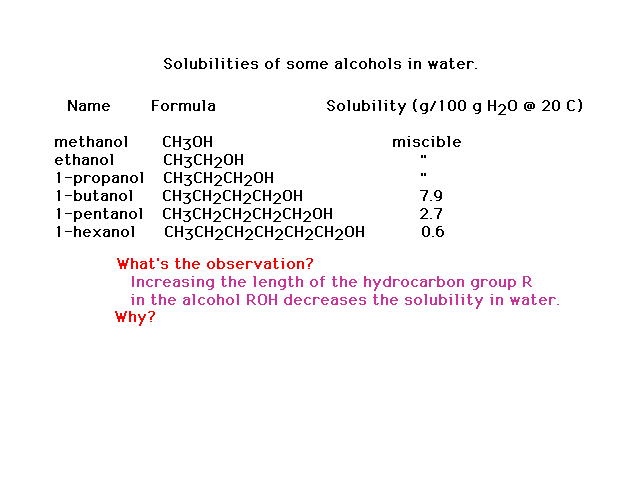 |
| Interpretation of alcohol solubilities in terms of
molecular structure and intermolecular interactions |
 |
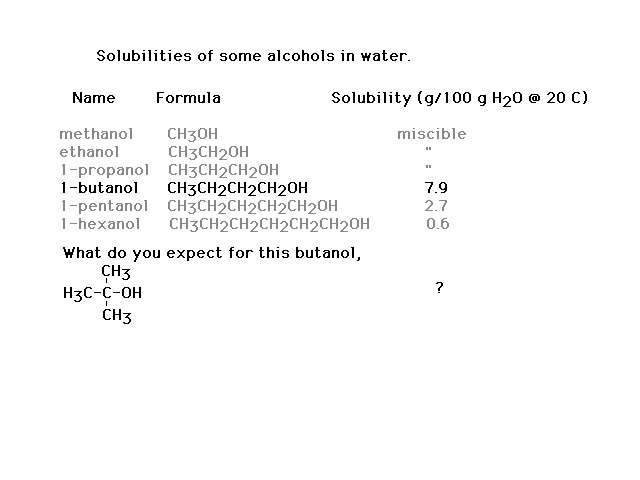
| Another comparison to make. |
 |
| Vitamin A shown here is essentially a nonpolar
molecule. It is very polarizable because of its
"size" (and also because pi electrons are very
polarizable, a detail we haven't worried about and will
not). Consequently, it is not very soluble in water but
quite soluble in a nonpolar solvent such as
"fat" (which is mostly hydrocarbon-like in
nature). |
 |
| In contrast to Vitamin A, you can see here from the
structure of Vitamin C that extensive hydrogen bonding
with solvent water molecules should be possible,
explaining why it is a water soluble vitamin. |
 |
| Acetic acid, as a small, polar molecule capable of
hydrogen bonding with water is very soluble in water. You
might reasonably expect it to be insolube in nonpolar
solvents. However, a subtle phenomenon causes acetic acid
to be soluble in nonpolar solvents as well. See below. |
 |
| Acetic acid can effectively "dimerize",
form a double structure held togeter very effectively by
a geometrical arrangement that accommodates double
hydrogen bonding. The resulting structure has no dipole
moment. The "dimer" can then serve as a solvent
in nonpolar solvents. |
 |
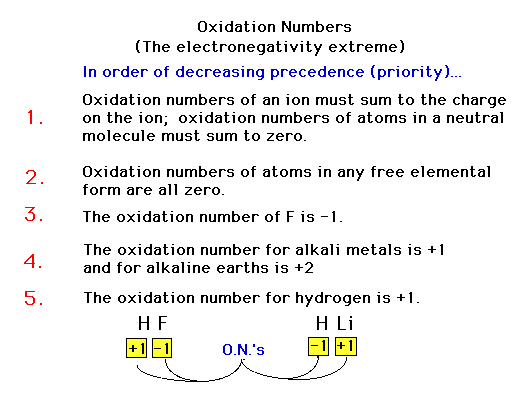
| Our rules for oxidation numbers. These are
"heirarchical", that is, each rule's rank
determines their importance relative to other rules. They
are not the same as in Table 4.3. |
 |
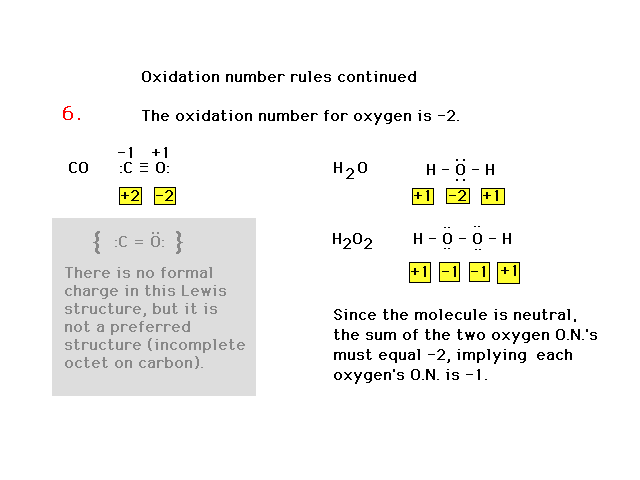
| Oxidation number rules continued |
 |
| Oxidation number rules continued |
 |



