| Lecture
#32 |
Text: Chapter 19,
sections 4 and 6.
|
| CURMUDGEON
GENERAL'S WARNING. These "slides"
represent highlights from lecture and are neither
complete nor meant to replace lecture. It is
advised not to use
these as a reliable means to replace missed
lecture material. Do so at risk to healthy
academic performance in 09-105. |
|
| Lecture outline |
Transition metal complexes
Stereoisomers
Optical isomers
Observations to explain
Colors
Magnetic Properties
Stability
Crystal Field Theory
|
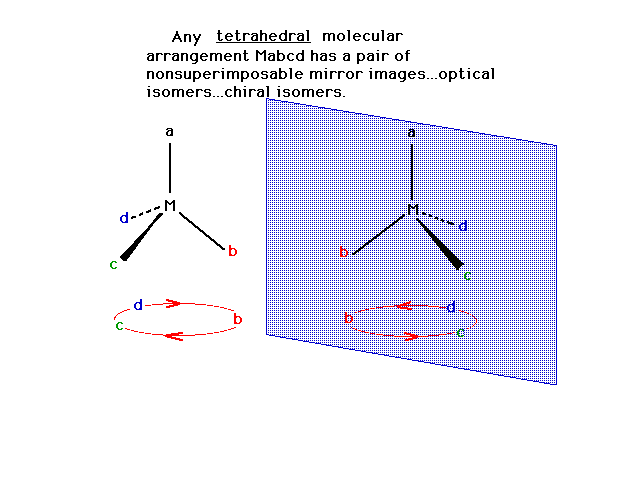
| A class of isomers called "stereoisomers"
consists of geometrical isomers, which we already have
seen examples of, and "optical isomers", also
called "enantiomers". Optical isomers are said
to be "chiral" and have a distinction between
them that is similar to the distinction between the left
hand and the right hand. |
 |
| A tetrahedral arrangement symbolized as Mabcd (four
different ligands) consists of two possible optical
isomers. 
|
 |
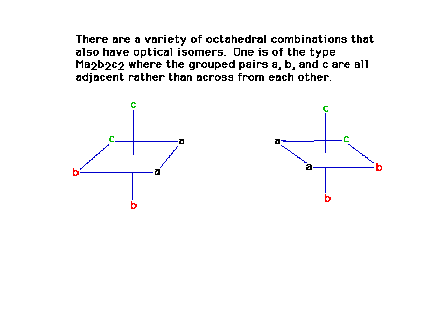
| The octahedral arrangement symbolized by Ma2b2c2
where the three pairs of ligands are all "cis"
with respect to each other has two optical isomers. |
 |
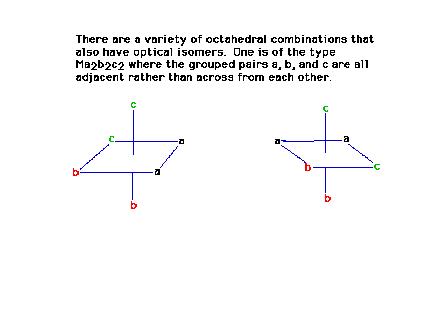
| The previous structure rotated so as to match up the
ligand pairs b and b with that of the mirror image, also
matching up one of the a ligands. The two structures are
not superimposible meaning that they then must be optical
isomers. |
 |
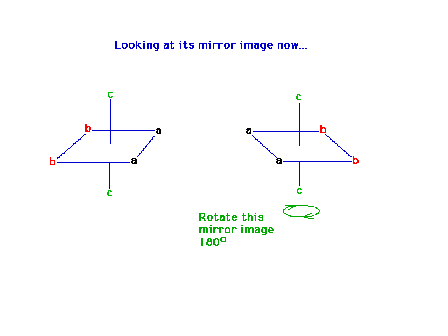
| The octahedral complex Ma2b2c2
in which the ligands are all "trans" to each
other is a geometrical isomer of the "cis"
arrangement. Its mirror image will be superimposible and
therefore there are no optical isomers of this structure. |
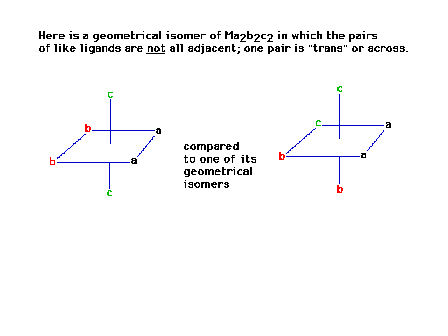 |
| The mirror image of the previous complex |
 |
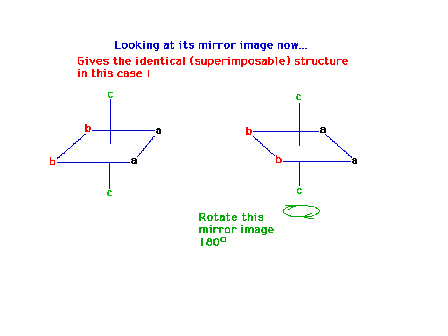
| The two structures (original + mirror image) are
identical. |
 |
| The electron configuration of the transition metal ion
Co3+ |
 |
| Light absorption properties of some Co3+
complex ions |
 |
| Magnetic properties of some Co3+ ions |
 |
| Geometrical properties of some transition metal ions. |
 |
| Crystal field theory addresses all the
"puzzles" from the previous lecture. |
 |
| Developing the idea of crystal field theory.. |
 |
| |
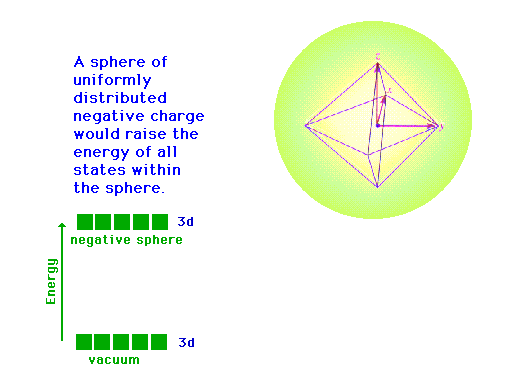 |
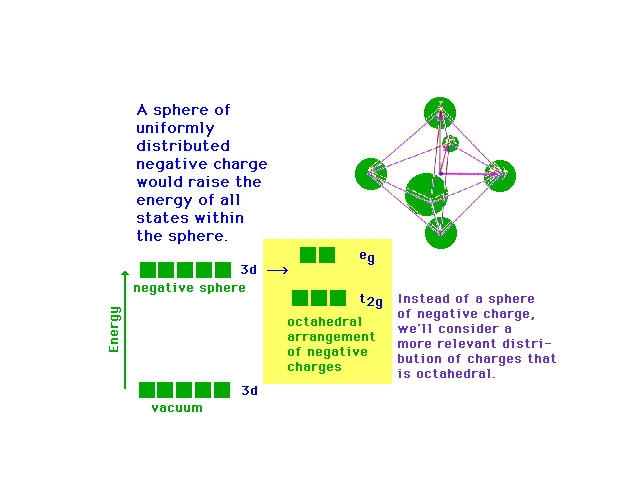
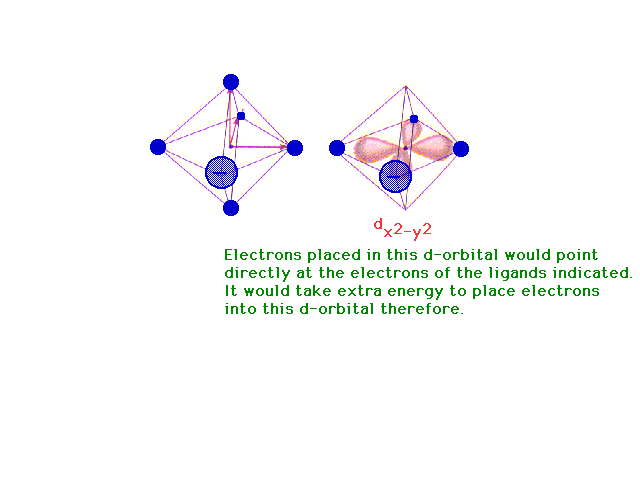
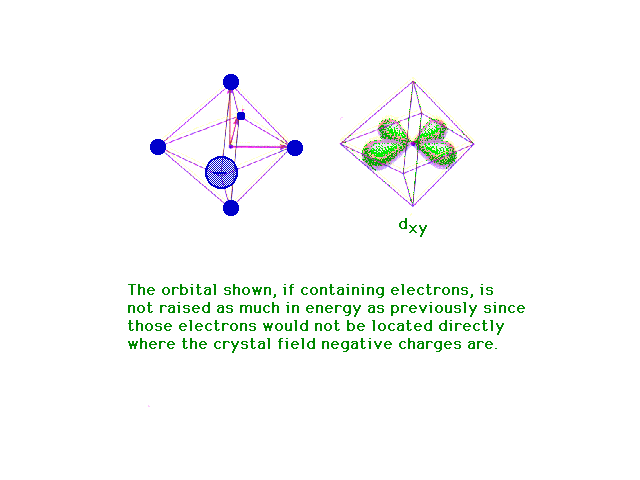
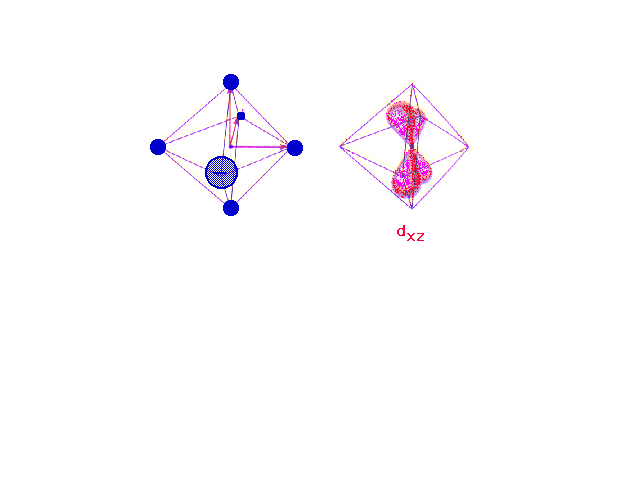
| Following what happens to the outer d-orbitals when a
transition metal ion is placed in a spherically
distributed negative charge, and then one that has
octahedrally deployed negative charges. |
 |
| |
 |
| |
 |
| |
 |
| |
 |
| |
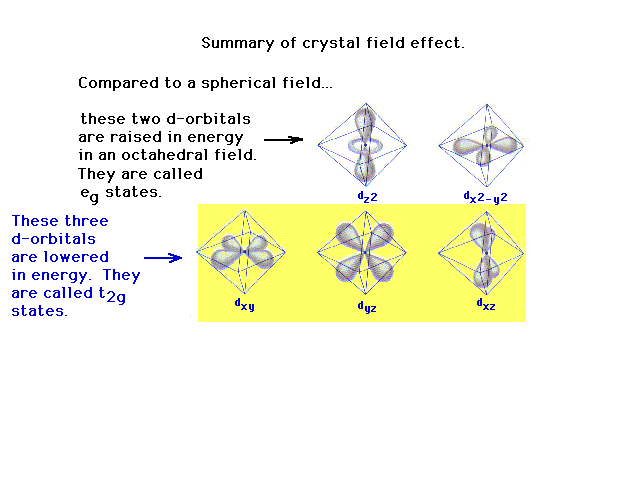 |
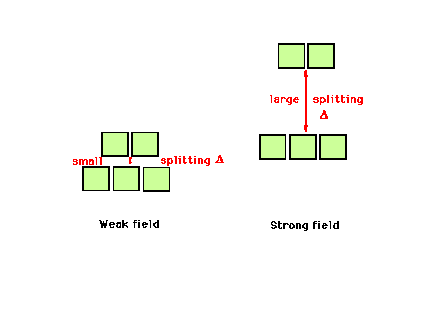
| The weak field splitting and strong field splitting
of the d-orbitals illustrated. |
 |
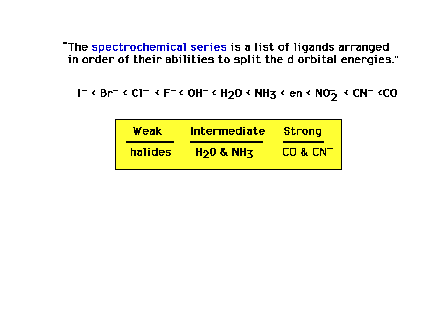
| You need to know the relative crystal field strengths
of a restricted number of ligands indicated here. |
 |
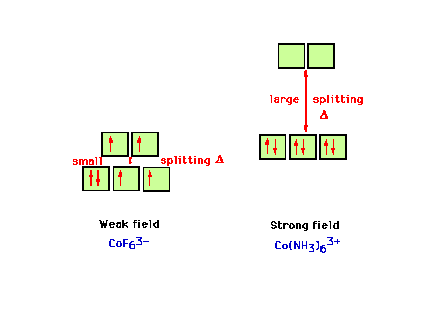
| The electron configuration in the weak field complex
CoF63- and the strong field complex
Co(NH3)63+. |
 |
| Co3+ complex ions, their crystal field
splitting energies, the color of light absorbed, and the
color that the complex appears. |
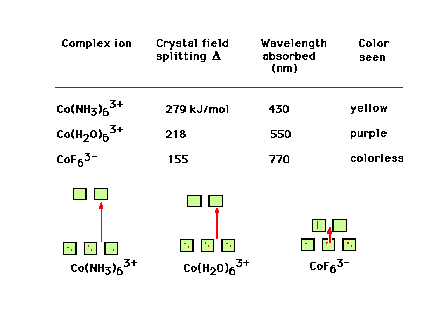 |
| Leaving the discussion of coordination number = 6
(octahedral geometries) and proceeding to coordination
number four. |
 |
| Changing an octahedral geometry into a square planar
geometry distribution of charges |
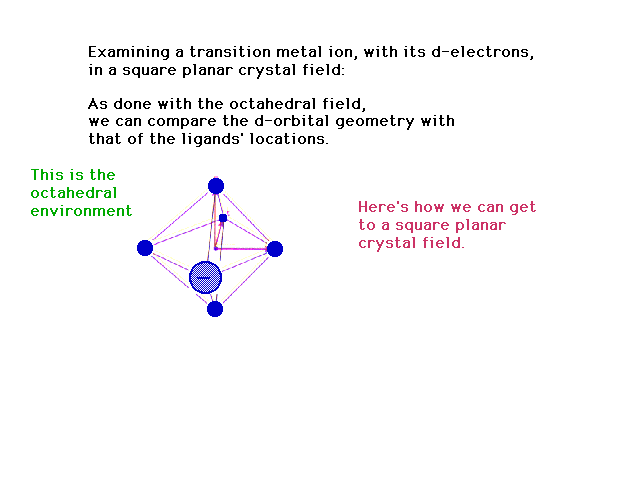 |
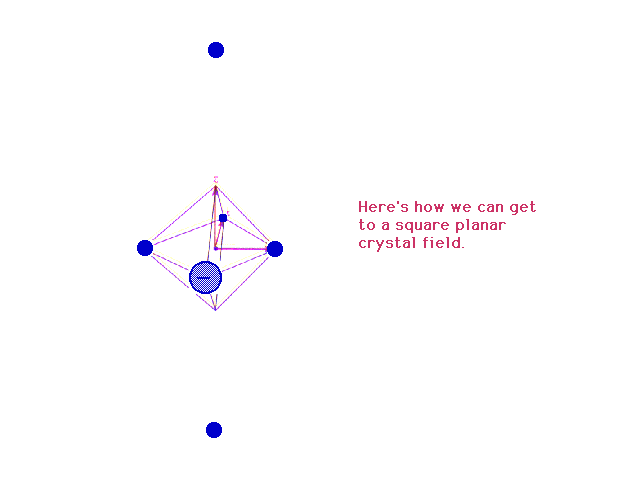
| Removing the ligands (charges) along the z-axis to an
infinite distance from the central species converts the
octahedral geometry to a square planar geometry. |
 |
| What kind of change to we expect the removal of
z-axis charges to have on the 3d valence orbitals? |
 |
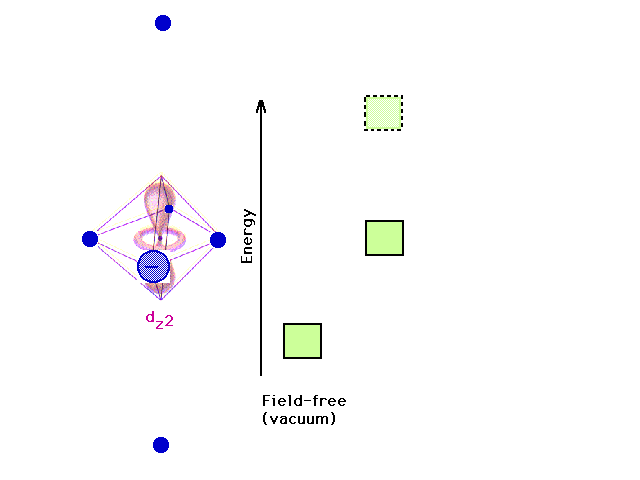
| Removing the z-axis ligands stabilizes the dz2
orbital relative to its previous energy. |
 |
| How are the remaining d-orbitals (shown in yellow)
affected by removing the z-axis charges? |
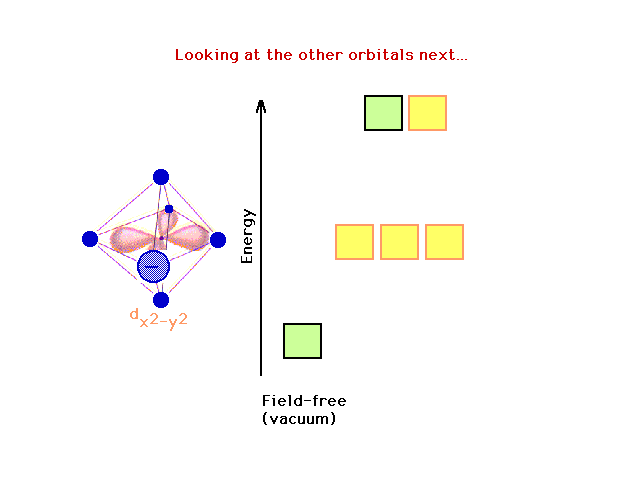 |
| Although the dz2 has been stabilized
(lowered in energy), there is no effect on the dx2-y2
orbital in changing something along the z-axis. |
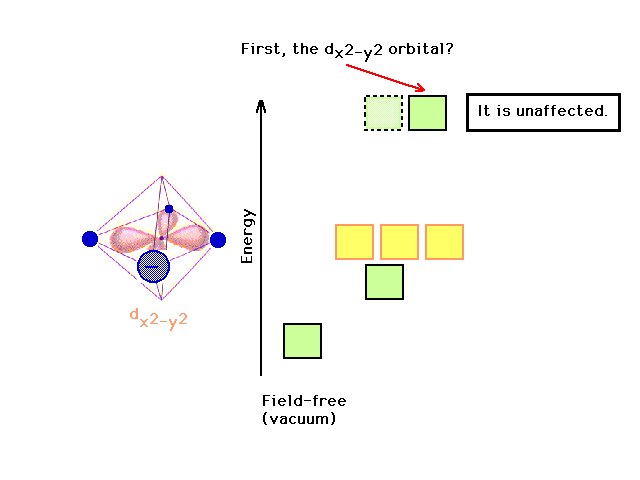 |
| There is also no effect on the dxy orbital
energy (now shown in green) of changing the charge on the
z-axis. |
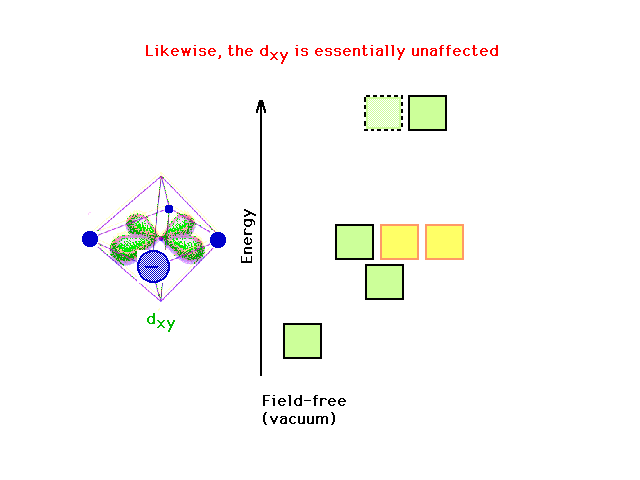 |
| Both the dxz and dyz are
lowered in energy (become stabilized) when charge along
the z-axis is removed. |
 |
| Summary of the switch to a square planar geometry |
 |
| The valence electron energy diagram for d-electrons
in a square planar geometry (crystal field). |
 |
| Now it becomes clear that the square planar geometry
correlates with the observation of extra stability of a
transition metal comlex ion with a d8
configuration on the central atom |
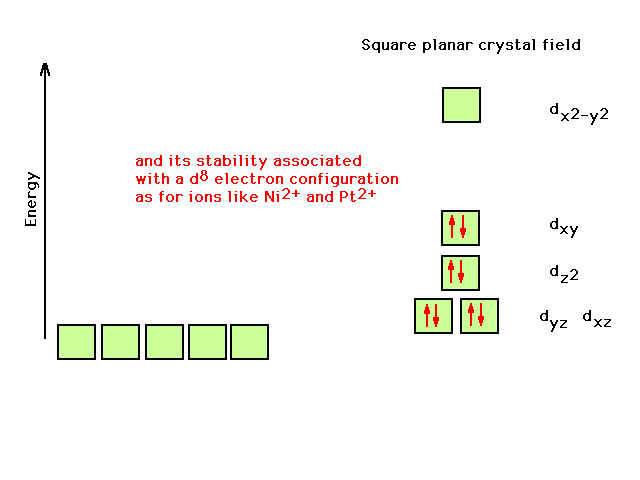 |


