| Lecture
#4 |
Chapter 12 (even though homeworks
emphasize
stoichiometry practice still)
|
| CURMUDGEON
GENERAL'S WARNING. These "slides"
represent highlights from lecture and are neither
complete nor meant to replace lecture. It is
advised not to use
these as a reliable means to replace missed
lecture material. Do so at risk to healthy
academic performance in 09-105. |
|
| DeBroglie's hypothesis of wave-particle
duality. This will lead us to the current theories. |
 |
| The wavelength associated with everyday matter (a
baseball) is so small, we don't see the effects of
wave-particle duality. Here, we see how the wavelength
depends on the total energy and the forces acting on a
particle, the latter showing up as potential energy
(which can vary with position of a particle in space). |
 |
| The next step was to take the equation for describing
how the amplitude of light varies in space (equation not
given yet) and convert it into an equation that shows how
a particle's wave amplitude varies in space. This is done
by using deBroglie's wave length for matter. We then get
a wave equation for matter. |
 |
| Lecture Outline |
(New Quantum Theory)
Particle-in-a-box model
one dimension
two dimenstions
three dimensions
Schrodinger's Wave Equation applied to the
hydrogen atom
n, principal quantum number
l, angular momentum or shape quantum number
ml, magnetic or orientation quantum
number
Electron "spin"
ms, spin quantum number
Energies and geometries
|
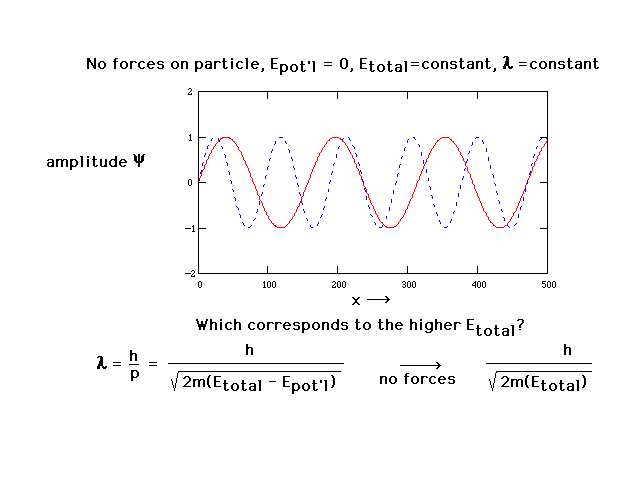
| With no forces present and total energy constant, the
wavelength is constant. This is now just a sine wave. The
amplitudes of two such sine waves are shown here. The
amplitude of a wave function has no physical significance
but will allow us to determine two features of systems
that we do have an interest in: geometry and energy (of
electron distributions in atoms and molecules). |
 |
| The particle-in-a-box model (Section
12.6 in the text), a very simple demonstration of wave
behavior of matter. We will need to understand this model
for 09-105. |
 
|
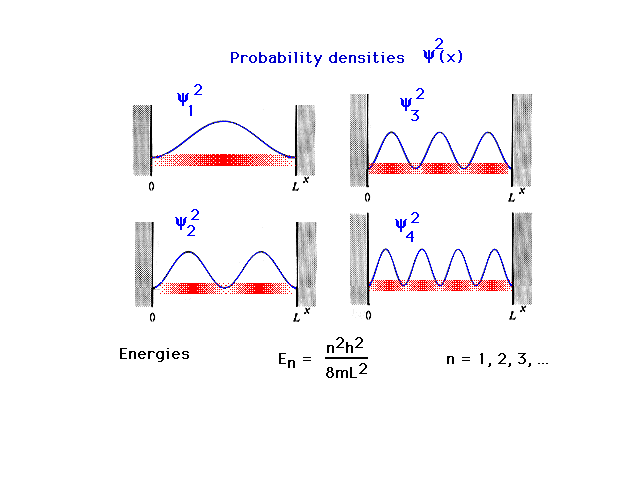
| Levels 1 through 4 for a particle in a
box; the wave functions or probability amplitudes must be
zero at the walls of the box. This restricts the
sinusoidal choices. |
 |
| The density distributions or probability densities
for the quantum mechanical particle-in-a-box are shown.
These are determined from the squares of the amplitudes.
The allowed energies of the particle follow a simple
formula involving an integral "quantum number",
n. These discrete energies are the only ones that exist
for this simple system. (The formula will always be given
to you. There is no need to memorize it for 09-105.) The
blurred density distributions tell you where you are
likely or unlikely to find the particle in each of the
energy states shown. |
 |
| The particle in a two-dimensional box |
 |
| The lowest energy state for the particle in a two-dimensional
box is describe by two integral quantum numbers. |
 |
| Wave function for the first excited state of the
particle in a 2D box |
 |
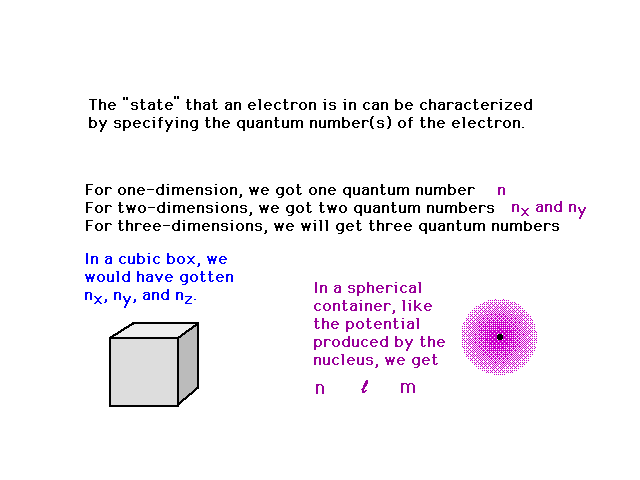
| We could go to a cubic three dimensional system
getting three quantum numbers (nx, ny
and nz). But let's jump right to the hydrogen
atom in which the electron moves in a spherical well
(produced by the central nucleus' positive charge). |
 |
| The principal quantum number, n, determines the total
energy of the electron |
 |
| The angular momentum quantum number will determine
the three dimensional shape of the wave function, how it
is distributed about the mathematical origin.
Restrictions on the allowed values of this quantum number
arise from the mathematics (which we don't look at in
this course). |
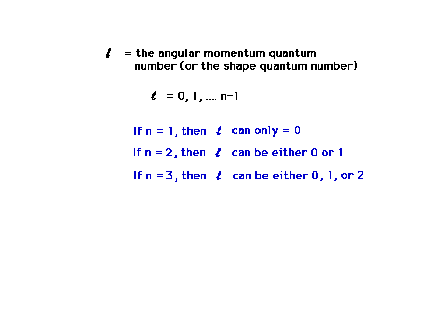 |
| There is an alternative set of symbols representing
the various angular momentum states of an electron. |
 |
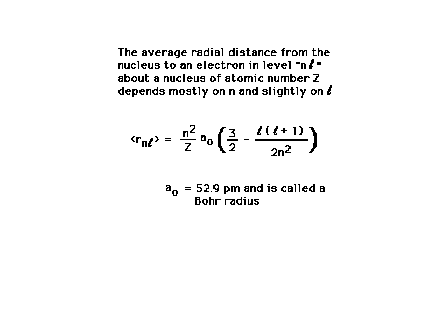
| The "size" of the region of space occupied
by an electron is difficult to quantify. The average size
depends mostly on n and to a lesser extent on the angular
momentum quantum number, l. (You do
not need to know this equation.) The essence of this relationship is very
well captured by Bohr's original formula for the radii of
his planetary orbits, rn = aon2/Z. |
 |
| The third quantum number is the magnetic quantum
number and refers to the behavior of an electron in an
atom if a magnetic field were present as well. Later
(when we're looking at pictures), we will find it also
indicates the orientation in which the wave distribution
points. |
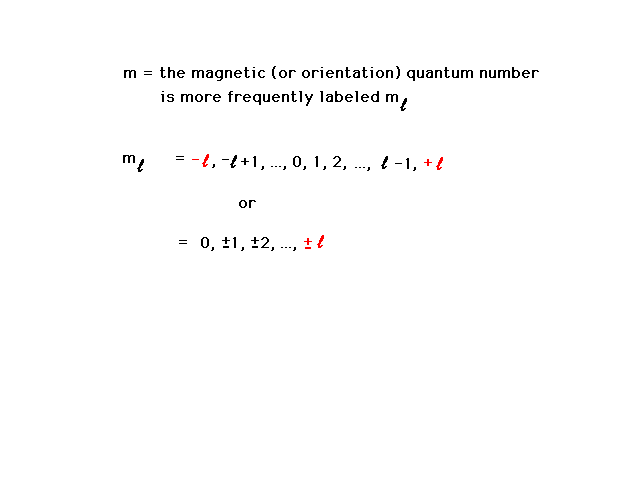 |




